Hepatitis C virus (HCV) is a single-stranded RNA virus that infects an estimated 180 million people worldwide.
In 2013, Gilead received FDA approval for a new HCV drug, Sovaldi (sofosbuvir), that inhibits viral replication by targeting the virus’s NS5B polymerase. Sovaldi has shown a very high cure rate (nearly 100% HCV suppression and sustained virological response) in clinical trials of previously untreated patients and has fewer side effects than pegylated-interferon and ribavirin therapies.
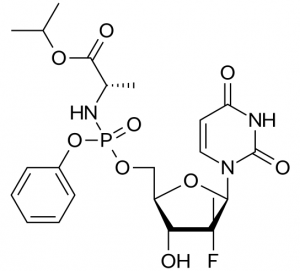
Sovaldi is a methyluridine-monophosphate prodrug: it is metabolized in the body back into methyluridine-triphosphate, which acts as a potent substrate mimic and inhibitor of the NS5B polymerase.
What is interesting about Sovaldi is the approach the scientists took to getting the inhibitor into the cell, relying on phosphoramidate prodrug technology that had been effectively used to develop anti-HIV drugs, but had never been applied before to this class of anti-HCV drugs.
During development, the researchers decided that they needed to deliver the charged methyluridine-monophosphate (rather than the neutral methyluridine) into the cell on the basis of two key observations: 1) the methyluridine triphosphate is the active compound against HCV NS5B polymerase, while the methyluridine alone is inactive (owing to very low conversion to monophosphate in vivo) and 2) the methyuridine monophosphated derivative can be anabolized in the cell back to the potent triphosphate form by an endogenous uridine-cytidine monophosphate kinase.

The phosphoramidate prodrug technology had never been applied to HCV inhibition until Solvadi.
The idea behind phosphoramidate prodrug technology is to create a membrane-soluble neutral prodrug derivative that can be metabolized in the liver by carboxylesterase-mediated cleavage and subsequent steps back to the monophosphate form.
The researchers applied the approach and after a significant amount of SAR investigation and PK/PD studies around the chemical composition of the phosphoramide substituents, they concluded that the structure of compound shown above was the optimal structure to deliver the methyluridine-monophosphate to the liver.
The result is a new generation of highly effective HCV therapeutics with few side effects that can make a significant difference in the lives of patients living with HCV.